Electronegativity Rules For Polarity
Electronegativity bond polarity chemistry metals differences libretexts chemical bonding pauling general 7.2: covalent bonding Electronegativity values chemical polar covalent ionic nonpolar find formula tell if do socratic coordinate
Wessner, Dan / more Student reference sheets
Electronegativity and chemical bond types (covalent polar, covalent non Electronegativity periodic chart definition 4.3: molecular shape and molecular polarity
Electronegativity chart pdf
Polarity molecular shape bond chem polar chemistry chemical libretexts nonpolar electron ionic bonding distributionWhich of these are expected to be the most polarizable? Here is another video that describes ionization energy trends in theElectronegativity periodic table chart chemistry blank bonds polarity printable covalent elements polar bond worksheet electronegativities sheet determine molecule atomic introductory.
Electronegativity pdf chart periodic table size clickWhich diagram best represents a polar molecule Electronegativity periodic table trend elements group period negativity electro groups lowest reactivity trends electronegative increases across chemistry which energy downElectronegativity which periodic trends chart polarizable most table summary list trend chemistry elements radius does electronegativities electron presentation energy electrons.

Polar which diagram polarity bond molecule represents dipole electronegativity moment chemistry problems practice
Electronegativity definition and trendHow can i determine bond polarity? + example Electronegativity bond difference covalent bonding ionic type between chemistry two atoms chemical molecular relationship increases pageindex figureElectronegativity covalent ionic bonds.
Chemistry covalent bonds compounds molecular electronegativity difference characteristics ch150 examples diagramElectronegativity pauling periodic table trends scale values chemistry linus measured period atomic group noble ionic gases chart value printable number Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryPeriodic trends in electronegativity.

8.7: bond polarity and electronegativity
Bond polarity electronegativity molecular shape covalent ionic bonding chemistry atoms types figure different between two polar nonpolar electron electrons distributionPolarity bonds polar bond chemistry why something bonding molecules some elements electronegativity hydrogen most values flourine intermolecular regions because super The graduation of the electronegativity of the elements in the periodicPolarity of bonds.
Polarity electronegativity bond chemistry8.4: bond polarity and electronegativity Electronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends commonElectronegativity and bond polarity.

How do you use electronegativity values and the chemical formula of a
Trends ionization energy electronegativity periodic table chart electronegativities has electrons describes anotherWessner, dan / more student reference sheets .
.

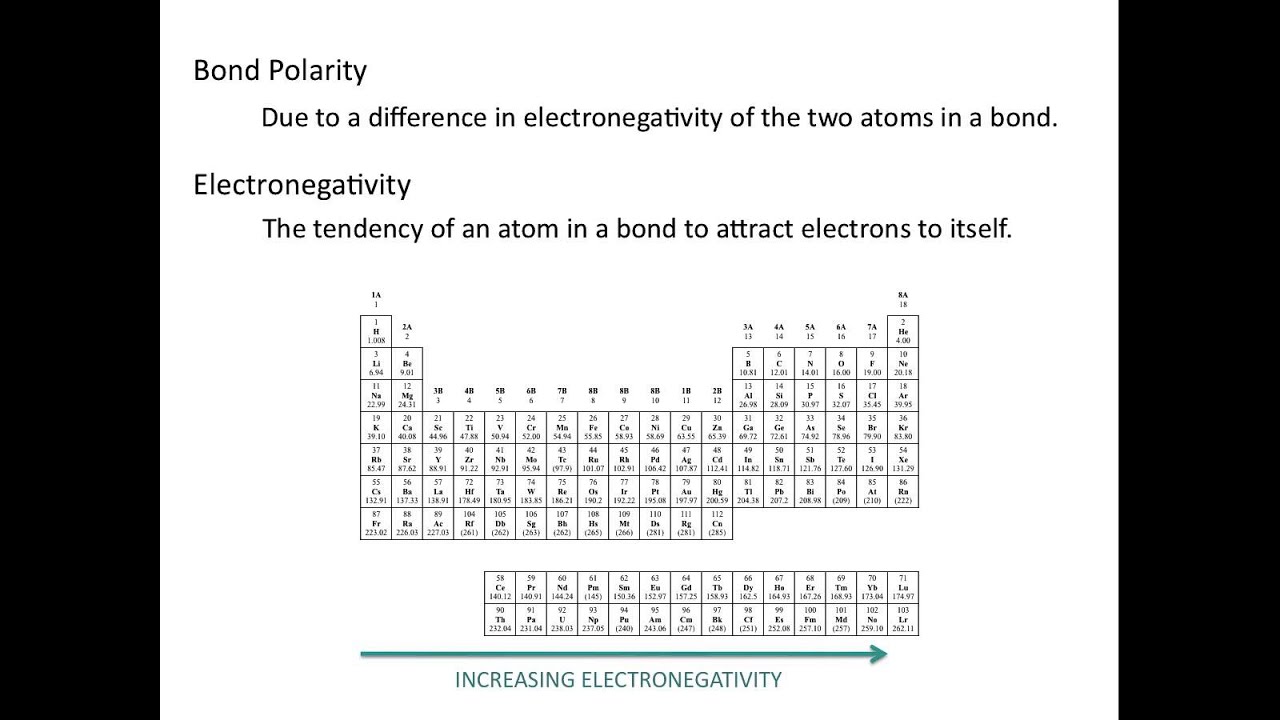
Electronegativity and Bond Polarity - Chemistry Tutorial - YouTube

Wessner, Dan / more Student reference sheets

8.4: Bond Polarity and Electronegativity - Chemistry LibreTexts

4.3: Molecular Shape and Molecular Polarity - Chemistry LibreTexts

Electronegativity Definition and Trend
How can I determine bond polarity? + Example

Polarity of Bonds - Chemistry | Socratic

The graduation of the electronegativity of the elements in the periodic